Panel tests catch the culprit, so to speak. In a situation where there could be many diseases causing symptoms, these tests find out which one. The more important it is to know, the more compelling the use case for the panel. A recent examination of EMRs of 11,000 Veterans Affairs patients highlights that COVID-19 is more dangerous than flu for hospitalized patients. Thus knowing which of the two diseases the patient has is important.
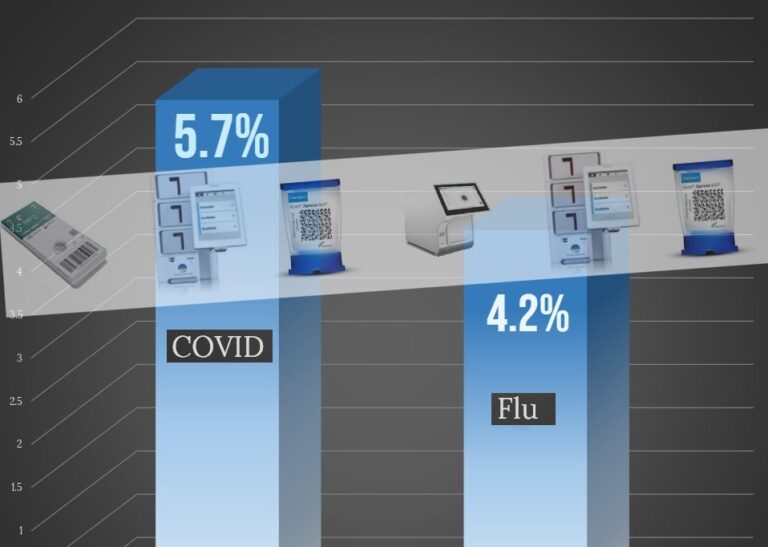
The study, conducted as it was in this most recent flu season, confirms this higher danger is true even in the post-pandemic era, though some talk was out there that the gap between the two diseases was lessening. The VA study indicates that among patients admitted for either ailment during this period, 5.7% of COVID-19 patients succumbed within 30 days of admission, whereas 4.2% of influenza patients did. This is as reported by Dr. Ziyad Al-Aly and colleagues from the VA St. Louis Health Care System.
This is a 35% greater risk of death in hospitalized patients for COVID-19 compared to flu. It’s important because there was a thought that this risk gap was reducing, but it hasn’t.
“We pretty much bought into the public narrative…thinking that COVID is no longer [more deadly than the flu], although there was no data,” Dr. Al-Aly said in an interview with MedPage Today.
Al-Aly, who heads up epidemiology at the VA Medical Center in St. Louis, has conducted earlier studies on both diseases with similar conclusions. “It seems flu is a more of a respiratory disease, while COVID is a multi-systemic illness; more organs are at risk,” (https://www.cidrap.umn.edu/covid-19/study-shows-long-covid-worse-patients-long-flu).
How does this play into respiratory panels? if all diseases are the same risk, and if the treatments are modestly effective, as they are with viruses generally speaking, why test? That more or less describes the approach in Western medicine until the development of syndromic panels and the theory of treatment around them. But once there’s a difference in severity, finding which you have will be critical for predicting outcome, choosing antiviral therapy and other steps.
NIRVs Before COVID
A little historical note: there was probably a need before COVID-19. Before the pandemic, respiratory diseases were categorized around influenza. That made sense because influenza led, but it wasn’t like it was the only condition out there. A study of US hospitals conducted from 2012-2015 found that while flu was the most common disease, 15% of patients had a non-flu respiratory condition (NIRV). [https://pubmed.ncbi.nlm.nih.gov/32056143/] Even before the pandemic, you saw interest in isolating some of those other respiratory threats, particularly RSV, and determining if more specific care or treatment would improve outcomes.
There are other respiratory diseases besides flu and COVID: Respiratory Syncytial Virus or RSV is the most common “third” disease of focus, for its tendency to cause lower-respiratory tract infections in both old and infant patients. There are other conditions not spoken of a lot but ER doctors see: adenovirus, human metapneumovirus, human parainfluenza viruses (despite its name closer to mumps than flu), bocavirus (mostly in infants and children), other coronaviruses.
Enter Respiratory Panels
Telling these diseases apart is made easier with a molecular (or a highly-senstitive immunoassay) test of multiple targets. IVD manufacturers were marketing such systems before COVID-19 and making a little headway, but the pandemic made differentiating between diseases necessary, especially as high COVID-19 caseloads remained during flu seasons.
As a result there are now many respiratory panels from most major companies: Roche, bioMerieux, Cepheid, Qiagen, Diasorin, and others. These tests can run for the respiratory Big Three: Flu, RSV and COVID-19. Companies reported fair sales of these tests and in our upcoming infectious disease IVD market report, we’ll talk about that. Whilst direct testing for COVID-19 was considerably reduced from pandemic levels, the disease will remain for some time as a panel test.
The respiratory panel is, it appears, the surviving product legacy of the pandemic boom in IVD.
While many panels are focusing flu (some with a A/B distinction), COVID and RSV, menus should increase. Quidel’s Solana panel RVP offers human metapneumovirus. Siemens Healthineers Fast Track RP-21 is a twenty-one-disease test panel, including all of those basic diseases, bocavirus, non COVID coronaviruses and parainfluenzas.
There are others, for instance: rhinovirus, the common cold. While not treatable, it is useful to know if cold is the culprit to rule out others. It’s also good to know if there is a comorbidity for patient assessment, especially children.
To the dollars and cents. It is a fact in the IVD market that a few core diseases make up the overwhelming revenue in the market. Hepatitis, Influenza, HIV are dollar volume leaders. But these diseases become more as a possibility on a panel. The respiratory panel is the surviving product of the pandemic boom in the IVD market.
The following is a rough list of respiratory panels
- QiaStat-Dx Respiratory Panel Plus
- bioMérieux SpotFire Respiratory Panel Mini
- Cepheid Xpert® Xpress CoV-2/Flu/RSV plus
- BD Respiratory Viral Panel-SCV2
- Roche Cobas SARS-CoV-2 & Influenza A/B
- Roche cobas® eplex Respiratory Pathogen (RP2)
- Diasorin Luminex NxTAG RPP
- Abbott Alinity m Resp-4-Plex
- Quidel Solana Respiratory Viral Panel (RVP)
- Siemens (former Fast Track Diagnosis) RP 21 Assay
- Panther Fusion® SARS-CoV-2/Flu A/B/RSV Assay
- Credo VitaPCR™ Flu / RSV Assay
- Lucira by Pfizer COVID-19 & Flu Home Test
So there are a lot of products, well, I will say a market research rule of thumb is: A plethora of products is a good indicator for a strong market. It doesn’t prove it, because products can come and go from the market, but generally more products more total sales, more demand. Whether the products cause sales or indicate sales is of less interest to an estimate-building market researcher, but I suspect there is some of both.
One More Thing: The Southern Hemisphere Season
Why discuss this in May? There’s a tendency in Northern Hemisphere nations (US and EU) to think of the flu season as “over.” But IVD is an international business. And the Southern Hemisphere, including the large markets of Chile, Australia, India, Indonesia and Brazil, feels its respiratory season now. Recent years the SH flu season has been a bit earlier, and last year its peak was in May instead of June where it had been in previous years. There are different vaccines for the Southern Hemisphere in most years, as those fickle flu viruses can change, but testing is run on the same equipment and with the same reagents generally.

Upcoming Infectious Disease Market Research Report
A reminder that our EYE ON IVD Infectious Disease IVD Market Review market research report is coming soon. Priced at $3800 for individuals and $7600 global site license, you will find it much more reasonable than alternatives whilst maintaining quality. That quality requires our checks and double checks, which is one of the reasons it is not yet released. It will be, prior to ADLM meeting this year.