When you hear a market figure (even from me) such as “the IVD market is $97 billion” it must be considered in all its dimensions. For one, many different individuals are receiving that revenue in some form, even if the lion’s share of it will be claimed by a few well-known names like Siemens or Revvity. There are landlords, there are employees (you could break out those by executives, salespeople and employees), there are shipping agents, there are distributors. Lately, retail outlets have been taking a share of home testing markets. But put these entities aside for a moment. They are accounted for as “costs,” and as all companies have them, are not that important in consideration of opportunities out there.
One cost factor that is an opportunity is contract manufacturing. The manufacturing of IVD creates two markets, the original manufacturer, the Roches of the world, and the third-party, or contract manufacturer. The first is understood, and my experience has been the second is not and requires a tool, resource, consultant, or some way of digging at the numbers to understand it.
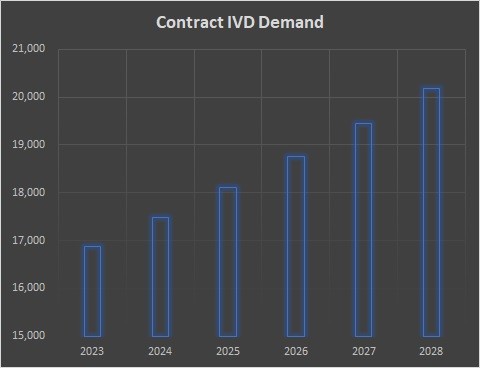
We estimate a $16 billion-dollar market for 3rd party contract manufacturing in IVD. We think it will grow to $21 billion by 2028, a little faster than the market for IVDs selling their services.
In IVD (in vitro diagnostics), we estimate about a $16 billion-dollar market in contract manufacturing of clinical testing products. [We will be releasing a Contract Manufacturing IVD Report in the Fall with greater breakouts of where all that revenue is coming from and which kind of test products are seeing contracting]. Now the first thing you should notice is, the market seems low. A 97 billion dollar market and just 16 billion is outsourced? Yes, that is likely true: Compared to almost every other major sector, medical devices have been slow to adopt contract manufacturing. Even today, the amount is paltry compared to say tech, furniture, or construction materials. There’s been an understandable and traditional fear of products leaving the company factory. What is true of medical devices broadly is especially true for in vitro diagnostics. The highly regulated nature of the industry is a factor in this traditional preference for in-company plants.
However, necessity has driven demand. The requirements of COVID-19 have only amplified the need for more contract-manufactured products. The global OEM in vitro diagnostic (IVD) manufacturing segment is well-positioned for long-term expansion. Various trends and factors will enhance the revenue potential of this business. Most leading global producers of proprietary IVD systems will increasingly rely on external contractors as a cost-effective strategy to diversify product lines, expand manufacturing capacity and capabilities, and cater to specific customers. Contract IVD companies will also see significant demand for producing private-label and non-branded reagents, test kits, and instruments for medical supply wholesalers and retailers.
Who are these companies that IVD marketers depend on to help develop products that meet the global demand for clinical tests? Some are IVD companies themselves, or owned by them. Examples of CMs include:
Here are examples of contract manufacturers:
1. Bio-Techne
2. BioKit
3. Charles River Laboratories
4. Lonza Group
5. Catalent
6. Patheon
7. WuXi AppTec
8. Covance
9. SGS Life Sciences
10. ICON plc
11. Eurofins Scientific
12. PPD (Pharmaceutical Product Development)
13. Samsung Biologics
14. Syneos Health
15. Emergent BioSolutions
There are many more. Hundreds really. What do they do? Services that could be offered for major IVD concerns include: customized quality controls, test development (an IVD is busy selling products and may want to hand over new test dev), wrapping and packaging, regulatory compliance support (only increasing in the wake of new regulations), technical assistance, plastic molding, A to Z final test production, liquid formulation and filling, powder preparation, powder mixing and filling, reagent tablets, freeze-drying (lyophilization), off-line/in-line labeling, kit assembly and packaging, and final product evaluation.
This isn’t a transparent resource. In my decade and a half of working with IVD markets, I received constant requests for this side of the market. It’s not impossible to develop tools, you just gotta sleuth a bit. Coming soon, EYE On IVD/Carlson Publications will be releasing our affordable and authoritative E-book on Contract Manufacturing in the IVD Market.
Stay tuned for our ADLM coverage. I’m gathering my pre-materials right now. But I can say, that one of the things we’ll be noting at ADLM this year is the amount and quality of contract manufacturers and their relative functions. It is often at least a third of the #ADLM convention, and we expect that to be similar in ADLM2024.